Natural Citral (≥85% Purity)
Pharma-Aligned · Traceable · GC/GC-MS Data · C-14 Compliance Ready
Designed for EU clean-label testing & regulated applications.
Specification Highlights
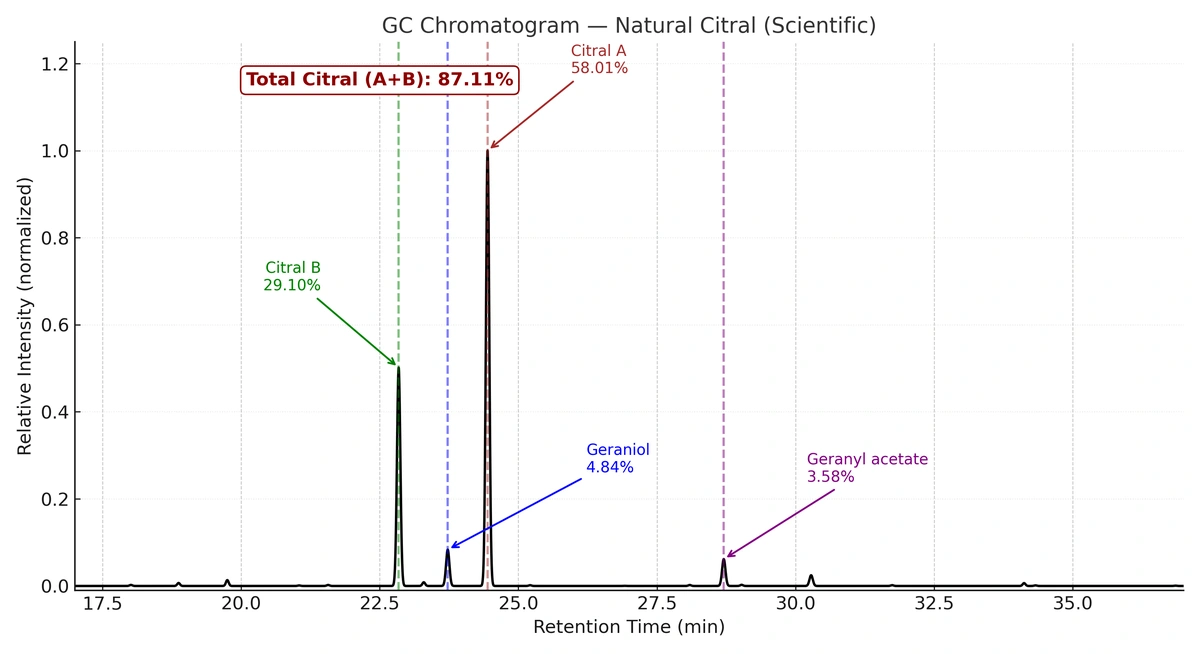
- Purity not less than 85% (average assay ~87.1%)
- Solvent-free, additive-free
- Manufactured under ISO-documented systems
- GC/GC-MS analytical data available on request
- C-14 Compliance Ready — designed for EU clean-label testing
- Packaging: 20 kg / 200 kg drums
Buyer Education — Natural vs Synthetic Citral
Natural Citral (plant-derived)
Solvent-free route with traceability for clean-label claims.
Lemongrass cultivation → Steam distillation → Crude lemongrass oil → Fractional distillation → Citral enrichment → Purification (solvent-free) → Natural Citral (≥85%)
Takeaway: Clean-label & regulated markets — validated by GC/GC-MS & C-14.
Synthetic Citral (petrochemical)
Multi-step chemical synthesis yielding high nominal purity but no natural origin marker.
Crude Oil → Propylene → Oxidation → Aldol Condensation → Prenol → Methylheptenone → Catalytic Isomerization → Citral (Synthetic, 95%+)
Takeaway: Lower cost, high-purity option — not clean-label; limited regulatory acceptance.
Product Matrix — At-a-glance
| Feature | Mansa Nutri Natural Citral | Synthetic Citral (Fragrance) | Synthetic Citral (Food) |
|---|---|---|---|
| Origin | 100% natural — lemongrass | Petrochemical | Petrochemical |
| Purity | ≥85% (avg ~87.1%) | 95%+ | 95%+ |
| Stability | High | Limited | Moderate |
| Food-use | Yes — COA & C-14 | No | Yes |
| Traceability | Farm-to-batch COA | Limited | Limited |
| C-14 (Radiocarbon) | ~100% pMC (natural) | 0% pMC | 0% pMC |
| Price | Competitive (natural premium) | Lower | Lower |
Innovation Pipeline — Future Molecules
🧪 Our R&D pipeline spans next-generation aroma and pharma molecules, engineered through biotechnology and advanced process design. Each candidate is benchmarked for pharma and flavor applications, with specifications aligned to global compliance standards.
Commercial readiness will be announced following validation and regulatory clearance.
Verified Documentation & Supply Assurance
Verified Documentation
- Sample COA — available on request
- GC / GC-MS data — provided to qualified buyers
- C-14 compliance — available per order for EU clean-label testing
Supply Assurance
- MOQ & lead time — confirmed on request
- Export readiness — IEC registered; full export documentation
- Global packaging — pharma-grade handling (20 / 200 kg drums)
- Check Pricing & Availability →
